The overexpression of DNA repair genes in invasive ductal and lobular breast carcinomas: Insights on individual variations and precision medicine
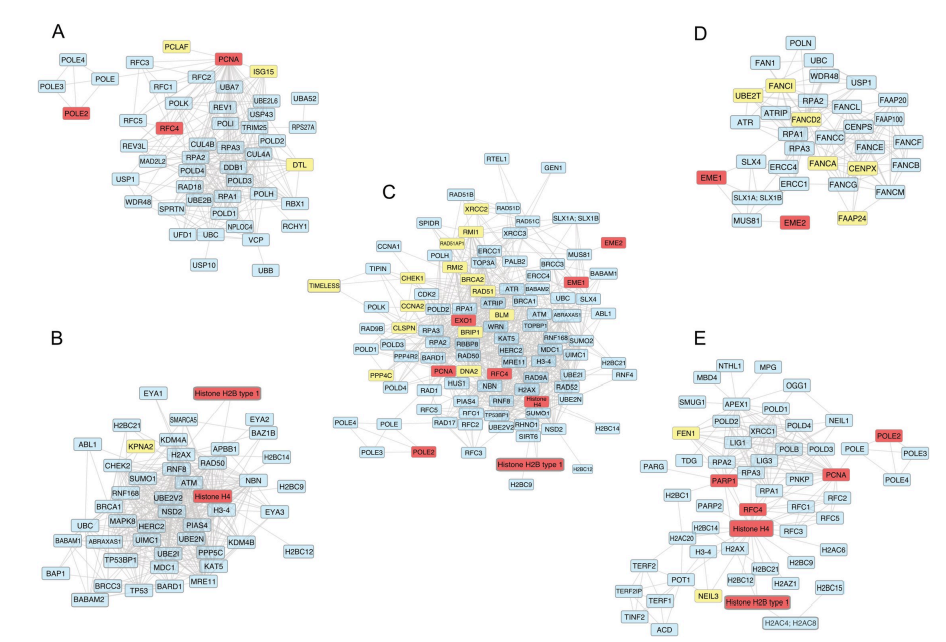
In the era of precision medicine, analyzing the transcriptomic profile of patients is essential to tailor the appropriate therapy. In this study, we explored transcriptional differences between two invasive breast cancer subtypes; infiltrating ductal carcinoma (IDC) and lobular carcinoma (LC) using RNA-Seq data deposited in the TCGA-BRCA project. We revealed 3854 differentially expressed genes between normal ductal tissues and IDC. In addition, IDC to LC comparison resulted in 663 differentially expressed genes. We then focused on DNA repair genes because of their known effects on patients' response to therapy and resistance. We here report that 36 DNA repair genes are overexpressed in a significant number of both IDC and LC patients' samples. Despite the upregulation in a significant number of samples, we observed a noticeable variation in the expression levels of the repair genes across patients of the same cancer subtype. The same trend is valid for the expression of miRNAs, where remarkable variations between patients' samples of the same cancer subtype are also observed. These individual variations could lie behind the differential response of patients to treatment. The future of cancer diagnostics and therapy will inevitably depend on high-throughput genomic and transcriptomic data analysis. However, we propose that performing analysis on individual patients rather than a big set of patients' samples will be necessary to ensure that the best treatment is determined, and therapy resistance is reduced. Copyright: © 2021 Mohamed et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.